I have felt physically better in the last week and a half than I have since starting the trial. That is a huge relief and I’m enjoying having some energy, stamina, and appetite.

and they were the best I’ve ever had. It’s wonderful to enjoy food!

When I got the REGN4018 at a 150 mg. dose, as an outpatient, for the first time in week five I was a little nervous. But I only had one low temperature and one round of vomiting in the whole week after, which felt minimal compared to every other treatment. Plus, my sister was on hand just in case I needed anything but I was shockingly o.k. and despite several generous offers from others to spend the night for week six, I felt well enough to be alone.
However, while this has been my most symptom-free week to date, I can’t say it’s been an easy-breezy week.
It’s been a challenging, confusing, and consuming week.
I’ll detail it here because what came up for me surprised me and it is the stuff patients should know can happen on clinical trials. Namely, I am talking about clinical-trial treatment changes, decisions, and errors.
And to be honest, I feel like I’m still absorbing, digesting, recovering, and moving on from the cluster f*ck that was last week. It’s like I’ve been on a rollercoaster ride without a safety bar, flying through ups and downs, highs and lows, curves and corners, and unable to see who is driving. Mostly, I’m holding on to anything I can grab, the seat, the car, my loved ones, and hope. I do not know if my experience of a phase one clinical trial is typical or not but here’s what happened recently.
On Friday, 3/18/22, two days after my first out-patient treatment, and one day after finding out I had a confirmed response to treatment with a 30% reduction in cancer, I felt good. I was enjoying fever-free life and not having to be in-patient or having to go to MGH in Boston for labs or appointments two to three times a week and letting the good news about having a treatment response sink in. However, Friday morning I woke to a from someone at MGH telling me my afternoon “tap” (draining of pleural fluid) was scheduled for later the same day.
“That’s a mistake,” I said, ” don’t have an appointment. Maybe it’s another patient or a miscommunication?”
But it was not a mistake. The clinical team at MGH had met earlier in the morning, discussed my case, and decided I should have a “tap” that day because the amazingly good news CT not only showed a reduction of cancer but also an area of trapped fluid or pus in my pleural space and they wanted to be sure my infection was cleared up before proceeding to my next treatment. They started to wonder how many of my fevers had been from the REGN4018 treatments, and how many might have been from the trapped (technical term “loculated”) fluid in my pleural space if that fluid was indeed infected.
I’d had loculated fluid in my pleural space since the PleurX was put in before the trial even started (at the end of January), so I wasn’t clear on when and why it was now an emergency. I had to get a PleurX put in to be eligible for the trial because the trial did not allow people who got “taps” (out-patient draining of the fluid) in the trial. Plus, I preferred to drain the fluid at home, myself, rather than having to go to treatments (which aren’t cheap even with insurance). So now, it seemed I was back where I started before the trial started, with a pleural effusion and without a PleurX (because it had been removed). However, the trial does allow patients to get a tap after we enrolled (just not before).
But I didn’t want to spend half of my Friday at the hospital. “This is such a buzzkill,” I told the clinical trial nurse. I felt like I didn’t even have a chance to celebrate or digest the good news before having another visit, complication, or issue. I complained a bit but got over myself and let the trial nurse know that I’d come in for the tap, and if it showed infection I’d take an antibiotic. However, I was clear that I didn’t want to take another antibiotic, “just in case,” because antibiotics can decrease the effectiveness of immunotherapy and increase the risk of colitis – which is already a risk for anyone getting Cemiplimab like I was supposed to be getting soon.
I will say that I have read lots of studies about antibiotics reducing the effectiveness of immunotherapy, as well as hearing the same from my naturopathic doctor, Dr. Gurdav Parmar. However, the nurse practitioner, nurse, and others all let me know that they never heard of that research, need to give antibiotics often, and if there were anything to it they would probably be in the know. To be honest, I think they felt I was overreacting to having to take antibiotics and in truth, maybe I was, but I actually do believe the science says antibiotics before an immunotherapy drug can limit how it well it works even if others do not. I don’t think I’m reading junk science or radical perspectives but if anyone knows other research showing that antibiotic use is not a risk factor for patients doing immunotherapy, please let me know. Here’s what I’m reading:

doi: 10.1016/j.ygyno.2021.01.015. Epub 2021 Jan 24.)
Early in the trial, I had complained of back pain bad enough to request a lidocaine patch, something I’d not needed with my first PleurX. I’d shared pictures of what looked like a green smoothie coming out of my PleurX drain, and said, “I’m afraid I have an infection,” because I’d never seen that color before. My concerns were minimized – at least that’s how it felt to me. But I was also happy not to be getting antibiotics so I didn’t press hard.



I was told the pain was probably from the REGN4018 which causes inflammation and can magnify existing pain and issues. And I was reassured that the green that came from my PleurX site, was also from the REGN4018 and a result of increased cytokines.
When I was an inpatient, the oncologist on the floor (not my regular gyn onc) and the resident checked my PleurX site and weren’t too concerned, and though my gyn onc was out of the office, he asked a colleague to check on me and the site and she also wasn’t worried about infection.
I kept saying, “I’m not a doctor, I know, but I didn’t think green anything is supposed to come from the body never mind the space around my lungs.” But I was happy not to need antibiotics. The next day, when I was in for routine labs, the clinical trial nurse asked me if she could look at the site (under the bandage rather than just looking around it like most had done), because she did not love how the images I had forwarded to the team looked.
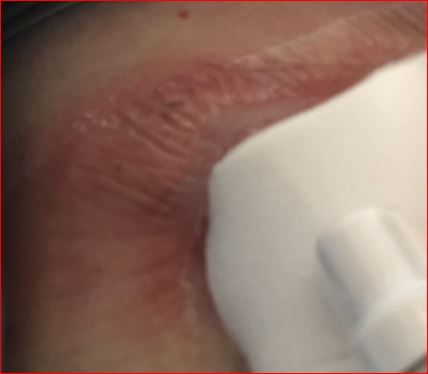
She was also concerned it might be getting infected and wondered if the PleurX should be removed? I was very much in favor of having it removed as I was no longer draining much fluid and it hurt a lot, like in the 5-7 range on a scale of 1-10. The clinical team worked hard making multiple calls and trying to get me squeezed in for a procedure by the end of the day.
“Can you wait a few hours?” my oncologist asked?
“Yes,” I told him as I was eager to get it out asap which happened later that day and IMMEDIATELY relieved my back pain.
Above, I said it felt like my back pain and worries about infection were minimized but that’s not entirely fair or accurate. The clinical trial nurse was the one who helped me get the PleurX removed and that was life-changing in terms of my ability to get comfortable and to be free of pain.
I’ve come to realize is that with new clinical trials, the medical team is in unfamiliar territory along with the patients. There have only been a handful of patients treated at MGH who are on the same trial. The medical team hasn’t memorized the protocols or rules. I don’t know if any other patients have had a pleural effusions or a PleurX when starting this trial, so my experiences are new to the team. They are also learning. They do not know yet how the REGN4018 treatment was impacting me, vs. what might be happening separately with regards to my PleurX because that’s not well-researched and they can’t compare me to other patients. The medical team is also in new territory during a trail, and they don’t always know how to make sense of our symptoms and what to attribute symptoms to.
When I think of how new, novel, and different a phase 1 clinical trial is, it’s a little intimidating. It makes me feel vulnerable.
VERY-VERY VULNERABLE.
On the other hand, the standard-of-care for #ovarian cancer is a standard-of-fail for most. New, novel, and experimental treatments are all that offer any real hope but they come with big risks.
I don’t live in the land of wishes, wants, and ideal options. I live in the land of making best of the hand I’ve been dealt given my circumstances. Instead of a big, wide, and expansive world there are times I’m simply choosing between what sucks the least. This is not unique to people with cancer. We’ve all had times when forced to make lemonade with lemons even when there’s no sugar to sweeten it up.

While the PleurX removal immediately eased all my back pain, it didn’t do anything for my fevers or vomiting. Plus, the site wasn’t healing fast or well. When my oncologist first told me the culture confirmed it was infected and suggested antibiotics. “Can I see if I fight it off?” I asked. I’d had an infection after my first PleurX was removal and this let the body fight the infection approach had worked. I hoped the same thing would happen and was grateful he was willing to let me try to avoid antibiotics.
For more than a week, I was draining fluid and pus requiring several wound changes every day. When what was draining from the wound began to smell bad I knew I was in trouble. So, even though I didn’t want antibiotics, the nurse practitioner insisted. While I shared how it might impact the effectiveness of immunotherapy she said something like sepsis isn’t good for treatment or outcomes either and I could not argue with that. Reluctantly, I did a full course of doxycycline.
But back to last Friday, March 18th, a week after that course of antibiotics had been prescribed, my sister-in-law was driving me to the T so I could labs and a tap, and we heard from the clinical trial nurse asking me to hold up because she was still waiting to hear back from Regeneron, the clinical trial sponsor, and she didn’t want me to make the trip into Boston for nothing. My sister-in-law and I sat parked in the car for a half-hour and there was still nothing from Regeneron per the clinical trial nurse. I asked if I should just come in anyhow. She wasn’t sure we decided to hold off til Monday or Tuesday since my next treatment wasn’t until Weds. I was relieved and thought it can’t be that much of an emergency if it can wait three to five days. Plus, my oncologist was out of town and out of the office at the SGO conference so the delay would give him time to weigh in on the best course of action.
My sister-in-law drove me home and I later heard from the clinical trial coordinator that the “tap” was scheduled for Tuesday afternoon, the same day as my echo, and before my Wednesday morning infusion. Later that night I got a text at 8:20 p.m. from my oncologist asking if I was free to talk. I panicked.
- Had my good scan results been an error?
- Why was he calling me at home over the weekend?
- Was I going to get kicked out of the trial?
It’s usually not good news when you hear from the oncologist over the weekend. But when we spoke, it was nothing to do with my scan results. Not really. My oncologist said he did not think I needed more antibiotics as the rest of the team did, but he wanted to make sure I was feeling well and was o.k. with not taking them (and I was). I told him I’d take antibiotics if the tap showed it is necessary but I didn’t want to start another course “just in case,” so I was glad he wasn’t insisting I do so.
The other thing he wanted to talk to me about is that my response to just the one Regeneron drug was clinically significant so I did have the option of staying only on REGN4018 and not escalating to the second drug – Cemiplimab. However, the trial sponsor left the decision up to my oncologist.
My oncologist shared that his “strong bias” was that I get the combination, to “go for the miracle,” but that he also knew there had been times when I said my body hit the limit and could take no more and he wanted to respect that. He mentioned the time I asked for a lower dose chemotherapy than he wanted to give me, and he feared undertreating me. However, even on the lower dose my platelets tanked to 20K (they are normally 150 to 400K, and surgery can’t be done if they aren’t at least at 50K), so it had been fortuitous that we didn’t go with a higher dose.
My oncologist also believes in and practices shared decision-making with patients which is one of the things I admire most about him. He gave me the weekend to think about my options. And at first I was annoyed and exhausted because it seemed all of my days were consumed with the trial. Even when I finally got fantastic news from my scan, there was no time to enjoy or celebrate.
Before the trial started I had endless tests, appointments, and intrusive procedures (get a port, get a PleurX, get a a CT, a PET scan, go to the eye doctor, the cardiologist and do many labs). And then the intensity of the trial was full blast from day one and the side effects made it so I was functioning in survival mode and leaning on family and friends to manage basic life. Then, there were complications and considerations as well and because the trial protocol requires weekly treatment – everything moves quickly.
For context, here’s a brief description from my patient portal of the trial I am on – NCT03564340:18-227:
.”… a phase 1/2 investigation of REGN4018 in combination with cemipilimab. REGN4018 is a bispecific antibody that targets MUC16 expressed on ovarian cancer cells and brings in the patients T-cells to attack the tumor. We reviewed that the most commonly seen side effects related to REGN4018 include CRS, abdominal pain, and ocular disturbances, and cemiplimab can be associated with autoimmune type side effects including, colitis, dermatitis, and pneumonitis, which in some cases can be life threatening. Cemiplimab (LIBTAYOTM) is a programmed death receptor-1 (PD-1) blocking antibody indicated for the treatment of patients with metastatic cutaneous squamous cell carcinoma.”
For a more detailed description of the trail, see the Regeneron website (here). It doesn’t seem to say what doses patients originally started and stayed at, (but I’ve heard 20mg of REGN4018 might be the max. dose for those who did the combination). However, I’m in the escalation arm of the study and so I do and will continue to get the full 150mg. It looks like the study goes for a little longer than six years and will enroll over 500 patients total (I don’t know how many of us have been in the trial to date). Below, are the most startling (to me) items being tracked during the study per the clinicaltrials.gov website:
- Number of deaths for REGN4018 monotherapy [ Time Frame: Up to 62 weeks ]Dose Escalation Phase
- Number of deaths for REGN4018 with cemiplimab [ Time Frame: Up to 62 weeks ]Dose Escalation Phase
These bullet points always remind me of the gravity and weight of my current situation which, for better or worse, I don’t obsess about over these things 24/7 because, I know, I have cancer but more so that as a mortal, we will all die.
To make decisions related to my treatment, and being on just one drug, or both, felt daunting because I’m not an oncologist or a scientist and no decision is guaranteed to be the right or best one. Part of me wanted to be told what to do but it was a small part of me. I kept reminding myself, “This is actually a gift” because I wanted an oncologist that treated me like a co-collaborator in my own care and that is exactly what I have. Sometimes, it means a level of responsibility that is intimidating but I prefer that to a doctor who doesn’t make time for patient questions, input, or concerns.
My oncologist doesn’t always agree with me but he always listens, responds, and explains what he is thinking or why he wants to proceed with one course of action over another. At the start of my treatment, I told my oncologist that I fear the loss of agency more than I fear the loss of life. I don’t know if that’s a survivor thing or just my personality but it doesn’t matter because it’s undeniable. I can’t imagine having a better oncologist or team to work with.
I spent a lot of the weekend consulting with my loved ones, doing research online, making pros and cons lists, and reaching out to the Clearity Foundation for treatment-decision support.

The Clearity Foundation shares resources and information about clinical trials, for free, to people with ovarian cancer. It’s an amazing service and one I trust. Their scientific director, Deb Zajchowski, Ph.D. (who is amazing and retiring soon though someone else will take her role), is not representing a drug company, a hospital, or a specific oncologist. She is sharing information and resources about clinical trials happening all over the United States and helping ovarian cancer patients like me get informed about our disease and treatment options. Her research and resources are helpful. Plus, she can explain things in lay terms. While Deb doesn’t give medical or treatment advice, she can help patients like me figure out the questions to ask our oncologists so we can make more informed decisions about our treatment/care.
She gets how difficult it can be to make decisions and knows as patients it will be us who have to live with/through (or suffer and die from) treatment complications and side effects. She doesn’t minimize patient personal experiences or our quality of life during trials.
I shared my experiences, questions, and concerns with the Clearity Foundation. I let Deb know that the trial had been intense, both in terms of symptoms and side effects, but also in terms of in-person labs, appointments, eye, and heart exams, costs, as well as multi-day in-patient stays at the hospital week over week.
I let her know that my liver counts had jumped 7 and 1/2 times and come down, that I’d lost 8 or more pounds in the first month, and that I needed a blood transfusion for the first time, None of these things had happened in the two and half years and three other treatment regimens I had been on. I reported that I waited almost a month for a fever and vomit-free day and was right up at the edge of what I could endure. I let her know my main worry was/is having cytokine release syndrome, especially since I’d had such a strong and early and robust immune response to the first drug. Here’s a bit about it from the www.stjude.org site.
What Is Cytokine Release Syndrome?
Cytokine release syndrome (CRS) is a collection of symptoms that can develop as a side effect of certain types of immunotherapy, especially those which involve T-cells. The syndrome occurs when immune cells are activated and release large amounts of cytokines into the body. Cytokines are small proteins that act as cell messengers to help direct the body’s immune response. However, high levels of cytokines may cause increased inflammation throughout the body. This can be harmful and interfere with a number of body functions. In severe cases, CRS can cause organ failure and even death.
These are all scary things to think about.
I also wasn’t clear how the low red counts would be managed, in the long-term, since my treatment is weekly. Would I always be severely anemic? Would I need monthly, bi-monthly, or weekly transfusions?
I wonder if there was something Regeneron knew about the drug combination, or my fevers, or my low red counts requiring a transfusion, that made them believe me escalating to the second drug might not be necessary. While my treatment team has only treated a handful of people in this trial, Regeneron hears from all hospitals and oncologists throughout the U.S. so they have more information about side effects than my team.
Is it typical to offered a change to a treatment arm during a Clinical Trial? Do oncologists get these calls often and if so are they required to share this information with their patients? I don’t know the answer to that but am grateful to have an oncologist who is transparent and doesn’t make decisions for patients but instead with us.
I seriously considered staying on just the one drug since I was finally starting to adjust to the medication. Maybe I didn’t need to take more risks, deal with more side effects and complications, and could just get benefit from one drug? Given how tired and beat up I felt from the first month of treatment, this sounded like a good idea to me. But, I wanted to do more research, because as my friend Beth says, “Your ovarian cancer is an aggressive MF,” and my oncologist told me that the patients he had who had responded best had the combination, not single agents, (though none was on the dose of REGN4018 I am on).
The Clearity Foundation consult helped me better understand the goals of the trial I am on and also helped relieve my fears about cytokine release syndrome. I learned that the in-patient stays are so MGH can identify and treat any cytokine release syndrome (or other reactions) but that this ability to detect and treat cytokine release syndrome is fairly new. While it wasn’t something that could be prevented, treated, and managed well in the past, it can be done now with close monitoring. That was VERY comforting information to learn because I know how serious and life-threatening cytokine release syndrome can be, how it can cause organ failure, and because of my fevers, I worried that I was at a higher than average risk.
The Clearity Foundation helped me understand the reason for combination treatment as well. In some cases, Deb explained, tumor cells can block an immune response to the first drug, and the second drug, Cemiplimab, may be able to stop (or reverse) that from happening somehow. However, Deb was clear that trials exist to test theories, expectations, and hopes to determine what is most beneficial for real patients (cuz theories can be wrong). She suggested I ask if anyone had a partial response to one drug, as I did to the REGN4018, or if the partial responses were only from the combinations. She suggested I ask if anyone else had a complete response to one drug or the combination as well.
My oncologist and the clinical trial nurse have been clear that their bias, preference, and belief is that both drugs, combined, are more effective than just one. “The combination is anticipated to have the best shot against ovarian cancer,” my oncologist wrote me, via email, adding “Both of my patients who did really well got the REGN4018 with cemiplimab but with lower doses of REGN4018 than you get.”
I ultimately decided that the best chance to fight my cancer is to do both drugs, to go for the 1-2 punch, and to be happy about the fact that I have had a good initial response but to remain as aggressive and pro-active as possible. I was nervous about the toxicities of a combination though. That’s not accurate. I was terrified and beyond exhausted.
I asked if there were any chance to delay when I started the Cemiplimab, by weeks or even months, so I could recover more before escalating and adding more drugs to the mix. While my oncologist was willing to ask if a delay were possible, he doubted it would be granted and he was correct. If I wanted to go forward with both drugs I would have to do so on treatment day.
So, I moved forward, had the “tap” done on Tuesday, March 22nd right before an echo, and was back at 7:30 a.m. on Wednesday 3/23 for my infusion. I came packed with clothes, food, and my laptop as I expected to be admitted. I met with the oncologist and the clinical trial nurse soon after I arrived and it was all systems go.

My infusion floor nurse came in the room and shared with the team something about being outside the window for adding Cemiplimab but the oncologist and clinical trial nurse weren’t worried.
After my oncologist left, he later texted me after we met to confirm I wanted to proceed, “Yes,” I said.
He texted to tell me to tell the infusion nurse that the orders were in.
However, even with the orders being in the nurse said she needed to wait to hear back from Regeneron before she could infuse the drugs without their permission because we were outside the stated treatment window. This made me worried because nurses always know everything about everything. So, the fact that my infusion floor nurse was nervous was enough to make me nervous.
So I waited. And waited. And waited.
Two hours turned to four hours.
My friend Beth joined me a little after noon, bringing pistachio nuts, love, and homemade banana bread. It was good to see her. She wanted to be with me when I got the first combination infusion, to offer TLC, support, and to get immediate help if I had an infusion reaction. She has come to most of my infusions (and there have been dozens), over the past two and a half years.
Together, we waited a few more hours.
Eventually, my oncologist came into the room.
“This can’t be good,” I said.
It was not good.
He told me I’d missed the window for starting the drug and could never get the Cemiplimab or combination drugs.
As he spoke, the clinical trial nurse came in, followed by the clinical trial coordinator, and then the infusion floor nurse. Mind you, we were in a room about the size of a bathroom and there were six of us standing or sitting and sharing the space. I was overwhelmed by the news. It felt like a body blow. Part of me was sad, part of me was mad, and most of me went numb. I was having trouble following the conversation and the words all sounded like blah-blah-babbling because I was dissociating.
- All that agonizing had been for nothing?
- I have no choice or say?
- How did I just “fall out” a treatment window without anyone catching me? No wonder it feels like a free fall….
- What’s the process for appealing?
But everyone just stared at me as I kept saying some version of “What-Huh-How?” and, “I don’t understand.”
Beth came over to hold my hand as the denial wore off. Her touch almost made me cry (but was so comforting).
The decision was final and firm. There was nothing I could do, nothing my team could do, and my only remaining option was to get the REGN4018 as a single agent, that day, and for the remainder of the trial.
My team asked how I was doing.
“It doesn’t matter how I’m doing,” I said, “Right? It’s done. So it doesn’t matter what I think or feel.”
I was trying not to cry or get angry while everyone was staring at me.
“It matters very much to us how you are doing,” my oncologist said. I believed his words but they didn’t feel true in that moment. I wasn’t ready to let them in – everything inside of me felt crowded and muddled.
They tried to cheer me by reminding me of my great scan results, my reduced CA-125, and the importance of a good attitude.
“I’ll get there,” I said, “But you all have been telling me the combination is best, preferred, most effective, most ideal, most potent against my cancer – including just this morning.”
I wasn’t ready to pretend that this was always the plan or a plan anyone else had even been on board with. I didn’t understand how something so major could happen to a patient in a clinical trial and how there were no safeguards in place to protect patients.
“Did they just f’up?” I asked my friend Beth when they all left the room. It took time for me to realize this was an error.
Had I been scheduled for treatment on Monday when the treatment window was still open, I’d have had the combination. Since I scheduled for Wednesday, two days outside the treatment window, I was out of luck.
But, to be clear, the treatment window had never changed or been vague. It had been defined in the treatment protocol but someone somehow missed it entirely so I was sh*t out of luck – not because of fate – but because someone made a mistake.
That took a while to wrap my brain around. And I kept going over it in my mind. How could this happen? There must be some alarm or process for monitoring deadlines. Was I at fault? Was the sponsor not clear? Was my team to blame? People don’t just fall out of treatment windows for no reason.
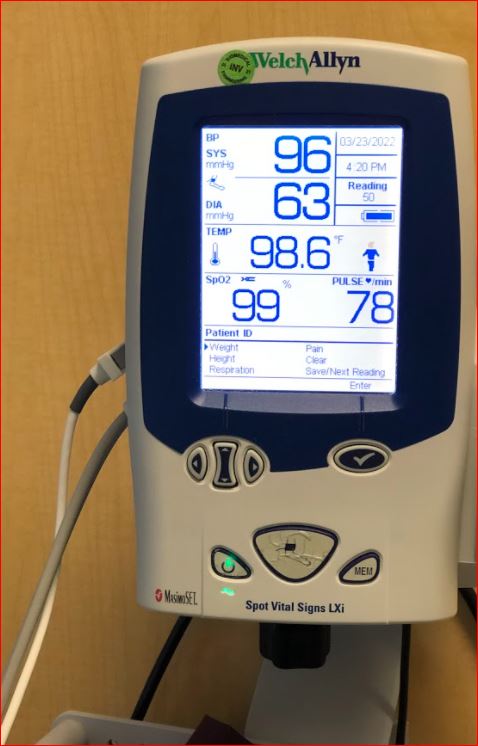
drug can make my blood pressure and oxygen saturation low, and my
temperature and heart rate higher. Luckily, the longer I’m on the drug,
even as the doses have increased, the more my vitals stabilize.
Mind you, my team is made up of people I respect, adore, and have a fantastic rapport with. These are the same people who have gone to bat for me in the past and who treat me with kindness and dignity. Still, did they not understand or know the protocol or the rules or the trial?
“It’s worth asking questions,” Beth said, “to get to the bottom of this just so you know.”
After I got the pre-meds, and the clinical coordinator came to visit, I asked if the issue happened because I had requested a treatment delay. She said, “No, not at all,” because the team had not pushed back my treatment.
I asked, “If I had always been insisting on the combination treatment, had never considered a single agent, or didn’t need a break, would this still have happened?”
“Yes,” she said, “unfortunately,” because the treatment protocols are usually strict. It’s not that exceptions are never made but it’s rare. Both she and the infusion nurse said there is a strict and complicated review process.
“Is it so the results are comparing apples to apples and not apples to oranges?” I asked.
Family and friends were texting me while we sat in the infusion room but I didn’t know how to respond quickly and clearl7. I was still adjusting to the change in plans, not just for that day, but for the rest of the trial.
“So no more in-patient stays?” I asked the clinical coordinator.
“No more,” she said.
“No more coming in all the time for labs?”
“No,” she said, and also shared that the number of scans and tests and screenings I would need would be much less as well.
It’s not like I can’t see benefits or find things to be grateful for. I was relieved to be done with hospital stays and from a treatment escalation I didn’t feel ready for.
I don’t believe everything happens for a reason but I do believe we can make or take meaning from most things and that there are unseen forces sometimes working on our behalf in ways we can’t or don’t understand.
I wondered:
- Maybe the universe had my back?
- Maybe my immune system would have totally overreacted?
- Maybe I would have been unable or unwilling to handle the toxicities?
- Maybe my red count would have dropped so low I’d have a heart attack?
- Maybe I’ll have a chance for another immunotherapy in the future and will respond even better when without an infection and while not being on antibiotics?
I could even find some humor and perspective in how things turned out.
The universe reminds us all we are not in charge, that all of my agonizing and research and discussions about over whether or not to stick on one drug or stay with two, are sometimes a waste of time. Decisions, even major ones, are not only by us but for us and sometimes as the result of human error, fate, or random circumstances.
I was trying to see the good in a situation that didn’t feel good or at least to accept that mystery.
Before the clinical team left the room after telling me I’d never get Cemiplimab, they asked if they could hug me. I hesitated. I care about each of them but I didn’t feel warm or cuddly or ready to hug it out. I was still confused-hurt-stunned and could only return half-hearted hugs.
The next day when my oncologist called to check on me, I asked why patients are not informed about these treatment windows and what they are. I told him I have a spreadsheet for daily symptom tracking my symptoms and I could have helped track the days, the window, and prevented this if I’d even known about it. He said some patients can partner and help police the trial rules but not all would want to. But many of us have apps on our phones or take pictures to track our temps, wound healing, and what we are going through. I have equipment at home to monitor temps, oxygen saturation levels, and blood pressure. It’s reasonable to at least give patients the option of tracking information and data.
Could the treatment window be shared with patients when we get all of the information and have to sign documents saying we are aware of all risks and possible side effects from experimental treatments? And why isn’t there an automated way to track patients, trials, and schedules? Where are the alerts, alarms, and notifications remind teams, especially when they meet right before a window closes, if someone is about to get disqualified from a trial? It’s not like I am an under-resourced hospital that has limited access to technology.
I am at MGH in Boston. I have a top tier team.
Again, a top tier team made of humans and sometimes they, like me, make mistakes. And sometimes those mistakes are huge. It doesn’t feel good to be the thing that fell through the cracks.
My infusion is warm, competent, and kind. She felt terrible for me because I’d sat in a tiny room for seven hours and had been given lousy news. She said that the hospital gave parking passes or a food voucher and she would request one for me. It was a kind gesture now I have a $16. credit at the MGH cafeteria for coffee and soup. However kind my infusion nurse was, a sincere apology from my treatment team would have been better. No one took responsibility for the mistake. Instead, I kept being told I fell outside the treatment window rather than the treatment protocol had not been followed accurately because it was misread or misunderstood.
My oncologist put this note in my patient notes:
“Sadly unable to get cemiplimab as outside the D36 window. I apologized that we didn’t know that before today as there was some confusion about +/- 3 days that does NOT apply to #1, and we do not think that she will be able to add Cemiplimab later, but we will ask.”
It sounds like I just sat in the wrong seat, the seat outside the window instead of inside the window and so, that would change the flight I was going on, the time it would take, and maybe even the destination as well. I felt powerless, and, a little insulted…. You can’t have the treatment your oncologist and team think is best, but here’s $16 worth of free soup for the less than optimal service. Oh o.k., I guess it’s all fine then.
I was a few coupons short of satisfied. What would have been more soothing is reducing my 2-3K medical bill for stuff insurance doesn’t cover or creating a system that keeps people from getting pushed out of treatment windows.
That said, I have a clinical team that cares about me and their patients. I have a team that is extremely patient-centered, responsive, kind, and competent. And human.
They are the same humans who have responded quickly to texts, calls, and emails, the same team who let me know there was an opening in this trial and tirelessly worked to get all of my appointments and screenings done ASAP so I could start treatment. They are the team I laugh and cry and share with and at least recently, see at least once a week but usually more often.
They are the team I will continue to work with, care about and trust going forward.
And I will make meaning out of this turn of events.

- I will take advantage of having more free time and a predictable schedule.
- I will enjoy feeling well enough to meet friends for walks and get out on the trails with my dog.
- I will get to make plans with my family members to do fun things.
- I will have conversations that aren’t just about symptoms.
- I will go back to the gym and keep rebuilding my strength and eventually be able to return to swimming.
- I will get over my infection.
- I will celebrate that I get these good days.
- I will return to my writing group.
- I will celebrate that my cancer is shrinking.
- I will be grateful I got to get in this clinical trial at all and that my medical team helped make that happen.
I may someday find out that the combination wouldn’t have worked for me or there’s some blessing in disguise I don’t yet understand. It’s possible. I may be one of the first people who do great on just one drug when it’s at a higher dose. Wouldn’t that be amazing?
I’m open to scientific advances and miracles.
And I know, sometimes we never get to know or understand why stuff happens. This might be one of those things I just have to live with. I can’t and won’t spend a lot of life being angry, sad, or irritated. I have to make the best use of my time. I will wrap my mind and heart around this and accept what has happened. I’m just not there quite yet. But I will get there and as soon as I do I’m going to go back to celebrating the fact that my cancer IS INDEED responding to treatment!

You Matter Mantras
- Trauma sucks. You don't.
- Write to express not to impress.
- It's not trauma informed if it's not informed by trauma survivors.
- Breathing isn't optional.
You Are Invited Too & To:
- Heal Write Now on Facebook
- Parenting with ACEs at the ACEsConectionNetwork
- The #FacesOfPTSD campaign.
- When I'm not post-traumatically pissed or stressed I try to Twitter, Instagram & Pinterest.
Hi Cis, thank you so much for detailing every bit of this so we understand exactly what you are going through. I hope your clear articulation of the medical error that prevented you from getting the two drugs (at least for now) is one that will be shared by your team and beyond. For what these studies are asking of the participants–the risks, the unknowns, the side effects–you outline some clear ways that the experience can be improved and I hope it gets acted on. Yes, you are lucky to be on this trial. Your team and the Clearity Foundation sound wonderful. AND everyone is human and there needs to be as many systems in place so that world-class medical experts do not get confused at crucial moments. We all want the same thing. Healing and eradication of cancer and yes, some miracles. There’s no reason we can’t get them. People walk on the moon and send machines throughout our whole solar system. Love you so much for your sharing and vulnerability and all your thoughts, fears, feelings, and research. You are helping more people than you know. I could not be more proud of you.
Dear Lynn:
Thanks for your feedback. A few people have reached out to me who have ovarian cancer and are interested in learning more about the study. That makes me happy. And also, I do think it’s not always clear what a burden and what a benefit clinical trials can (it’s often both/and). And you are right, we need systems in place that protect patients but also staff members because we humans are always going to be making mistakes. But, there should be safety plans and back-up plans so that things are caught BEFORE hand instead of when it’s too late. Thanks for your support and also for recognizing that the Clearity Foundation is an amazing resource and that my team is amazing as well!
I’m so grateful for you, for all the support, caring, and free-writing!!!
Warmly, Cissy
Thank you for sharing this story. You write so very well and express the emotional side as well as the factual side of things as they developed. It’s such a human story filled with suspense. I’m so glad to hear you’ve had a positive response so far and I am hoping and praying for the best for you
Thank you so much!!! I appreciate your comments, hopes, and prayers!
OMG, what a turn. Twist. Not your fault but damn, I want to blame someone! What good would that do? So, it looks like you are not going down that hole. Congratulations on your wisdom to not. And you are in control of the shrinking. It is happening!
Margaret:
I did go down that rabbit hole but I just couldn’t stay or live there for long! I hope you (and Ruby) are well!
Cis